Johnson Lab
Welcome
Our lab is located in the Institute for Quantitative Health Science and Engineering at Michigan State University in East Lansing, MI. We are affiliated with the Departments of Pharmacology & Toxicology and Biomedical Engineering.
What we DO
We leverage the versitility of digital manufacturing to construct complex in vitro models (CIVMs) for drug safety and chemical testing.
Why we do IT
Our goal is to develop and apply human-derived cell culture technologies that lead to the prevention of birth defects and disease in vulnerable populations.

The Complement-ARIE Challenge prize winners have been announced!
We just won a $50,000 prize!!!
Jacob Reynolds (BME) helped drive the proposal based in part on his research. The Prize Competition offered $1,000,000 in total prize money to diverse teams with ideas for new ways of using NAMs to conduct basic research, uncover disea se mechanisms, and translate knowledge into products and practice. Concepts from the winning entries of the Complement-ARIE prize challenge will be incorporated into the ongoing planning process for the NIH Common Fund’s Complement-ARIE program. Sudin Bhattacharya from IQ @ MSU also collaborated on the proposal. We are investing the prize money in our own research to help prevent chemical induced birth defects.
VIEW THE WINNING SOLUTIONS!


Student Rotations
Incoming 2025 BMS and BME PhD candidates, contact us about rotations
Currently funded projects in developming and/or applying complex in vitro models to test chemical disruption of energy metabolism and thyroid hormone imbalance. Rotation projects are hands-on and typically touch on all aspects of the lab, that is making an existing device, constructing something new (the new should be fun), repeating an experiment that works and testing a new hypothesis. Don’t worry, we’ll guide you along the way! Send bjohnson@msu.edu an email with student rotation in the header and we can discuss a potential rotation.


Latest News
Check out some of the latest news from the lab!
Birth Defects Research and Prevention in Denver
Conying was selected for a talk and recieved a travel award to the meeting.
Read moreGraduation!!!
Jacob Reynolds PhD, Sophia Caron BS and Dhruv Singh BS graduated in 2025! So proud of this crew, Jacob is moving to work in industry at ToxStrategies, Sophia to Indiana[…]
Read more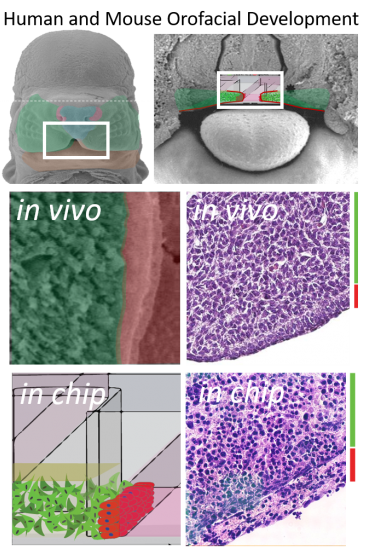
Biology driven design.
Intercellular signaling drives early development. Early organization of the embryo is orchestrated by just a few pathways including the Wnt, Tgf-B, SHH, FGF, and Notch pathways which often act as a means of cell/matrix or cell to cell communication. We engineered a device to phenocopy the developing facial processes (epithelial cells overlayed onto 3D mesenchymal cells) since these are sensitive to both genetic and environmental insults during development leading to facial clefting (cleft lip/palate). The resulting microtissues show critical similarities to tissue sections from the developing palate. More importantly, they support the spatial signaling interactions that are sensitive to teratogenic insults. We hope to use these microtissues to identity genetic risk-factors and chemical exposure combinations that may cause this birth defect in every 700 live births.
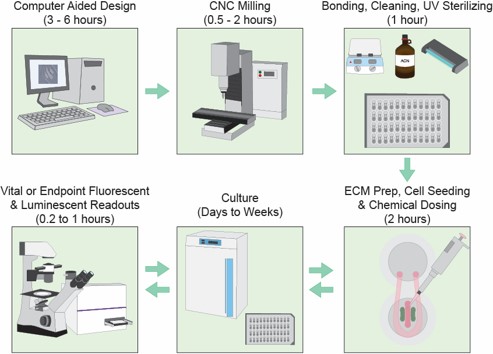
Digital manufacturing fuels innovation.
We developed a novel digital manufacturing process dubbed “microplate micromilling” that integrates microfluidic channels and features directly into standard commercially available polystyrene cell culture plates (48, 96, 384, 1536 etc.). This platform is used to generate tractable, adaptable, high content and throughput compatible intercellular signaling models.

Publications
See a full list of publications on Pubmed using the link below or check out a couple recent articles on the right.
My Bibliography
Meet the Lab!
We are an interdisciplinary group with expertise in the biological sciences and engineering.

Brian Johnson PhD
Principle Investigator

Keri Gardner MS
Lab Manager

Xiaopoeng Chen PhD
Postdoc Analytical Chem

Congying Wang BS
PharmTox PhD Candidate

Dhruv Singh
BME PhD Candidate

Vimbai Chado
MechEng Undergraduate

Evan Malbouef
MechEng Undergraduate
Amritha Yelleti
Biology Undergraduate

Jacob Reynolds PhD
2025 BME PhD Graduate

Sophia Caron BS
2025 Graduate on to Indiana for PhD!
CASE STUDY
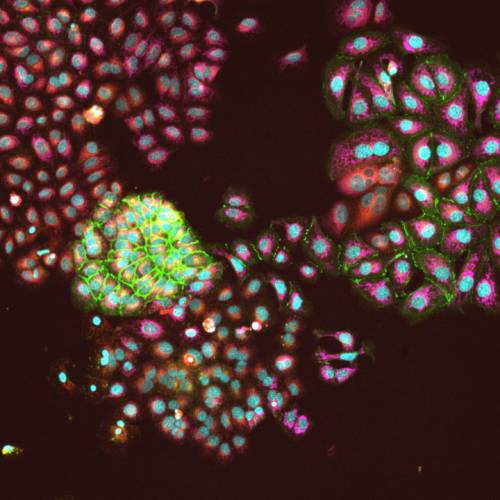
High-content confocal imaging identifies cellular heterogeneity
Image of Ishikawa cells imaged at 10x show hetergeneous cells in simple monoculture. Our high-content imaging pipeline streamlines imaging, image segmentation to identify individual cells and quantification of stains within each indifidual cell. The imaging cytometry work generates millions of datapoints to feed AI based learning and other quantitative analyses.
Equipment
Imaged with 10X objective on our Yokogawa CQ-1 high-content confocal imager.
Stains
Cells stained with Hoechst (nuclei/blue), wheat germ aggluten (membrane/green), nile red (lipid) and CellRox (reactive oxygen/fucia).
Connect with us!
Brian P. Johnson PhD 1- 517- 353–2541 bjohnson@msu.edu
775 Woodlot Dr Rm 3315 IQ Bioengineering Building East Lansing, MI 48824 Rm 3315 IQ-Bioengineering 775 Woodlot Dr East Lansing, MI 48824